Our Long COVID Research
|
We have done two small preliminary studies of our Axial Stability Method (ASM) Long COVID protocol, in partnership with researchers at Life University.
|
Researchers
Lydia Knutson, DC Lydian Chiropractic, Cambridge, MA Stephanie Sullivan, DC, PhD Director of the Center for Chiropractic Research at Life University, Marietta, GA |
Jennifer Massa, ScD Research Scientist, Harvard T.H. Chan School of Public Health Martha Herbert, PhD, MD Higher Synthesis Health |
Study Design and Purpose
Both studies were single-group exploratory studies, done without control groups. We simply provided the ASM Long COVID treatment protocol to a group of patients, and gathered systematic data before, during and after care. Both studies were approved by the Life University Institutional Review Board.
The purpose of the studies was to prepare to do a larger controlled clinical trial of the ASM Long COVID care protocol, by exploring various outcome measures. We gathered data on patient-reported outcomes, physical performance tests, and objective physiological measures, to discover what signals emerged in the data.
The purpose of the studies was to prepare to do a larger controlled clinical trial of the ASM Long COVID care protocol, by exploring various outcome measures. We gathered data on patient-reported outcomes, physical performance tests, and objective physiological measures, to discover what signals emerged in the data.
Participant Selection
All study participants were new patients at Lydian Chiropractic who had never been treated by Dr. Knutson nor received ASM chiropractic care prior to the study. Participants responded to Facebook ads and word of mouth. All participants had a diagnosed COVID infection at least 3 months before qualifying for the study, and had at least two Long COVID symptoms that could not be explained by an alternative diagnosis. They did not take medication to counter Long COVID symptoms during the study, and did not have other chronic diseases.
Demographics
- 13 participants
- 10 Females, 3 Males
- Age 30 – 57 (mean 47.2; median 49)
- BMI 22.2 - 42.0 (mean 28.7; median 25.7)
- Length of Long COVID symptoms: 3 – 25 months (mean 10.8; median 8)
Chiropractic treatment
- All participants received the same treatment protocol
- All chiropractic treatments are low-force (no manual manipulations of joints)
- Treatment was 15 – 26 visits (mean 18.1; median 17)
- Some participants had extra visits because injuries and other unrelated health events necessitated additional care that was not part of the Long COVID protocol.)
- Course of treatment occurred over a 2 - 3 month period
Patient-reported outcomes
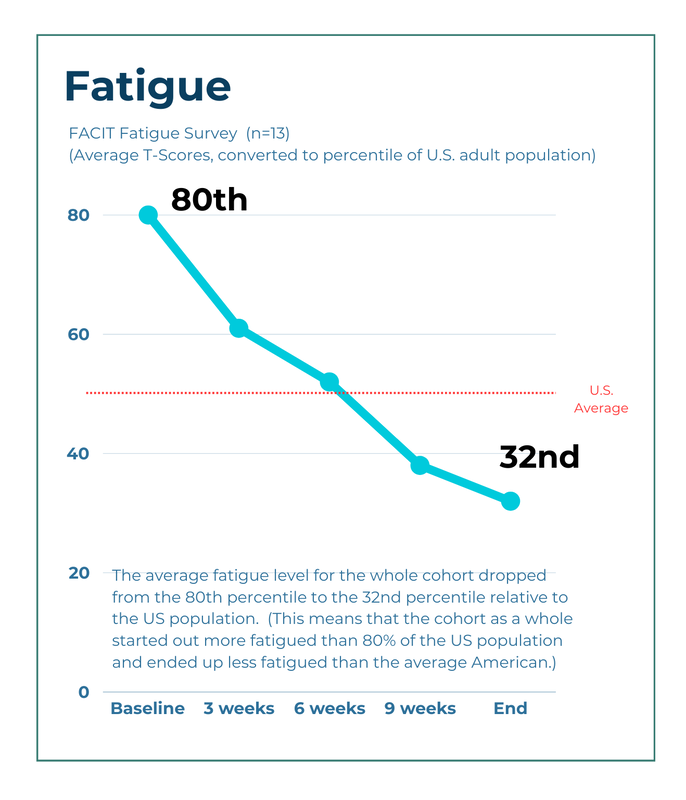
Fatigue We measured fatigue with the FACIT Fatigue Scale. The FACIT is a 13-question survey that is designed so its scores correspond to the fatigue levels of the U.S. adult population. Participants completed this survey each week during the study. The graph below shows the average T-scores, converted into percentiles, of our 13 patients at 3-week intervals during the study. |
Other Symptoms
Participants were given a list of 40 common Long COVID symptoms, and rated the severity of each symptom they had over the previous 7 days, on a scale of 1-10. They completed this symptom survey 4 times during the study: before treatment started, and after phase 1, phase 2, and phase 3 of the ASM Long COVID protocol.
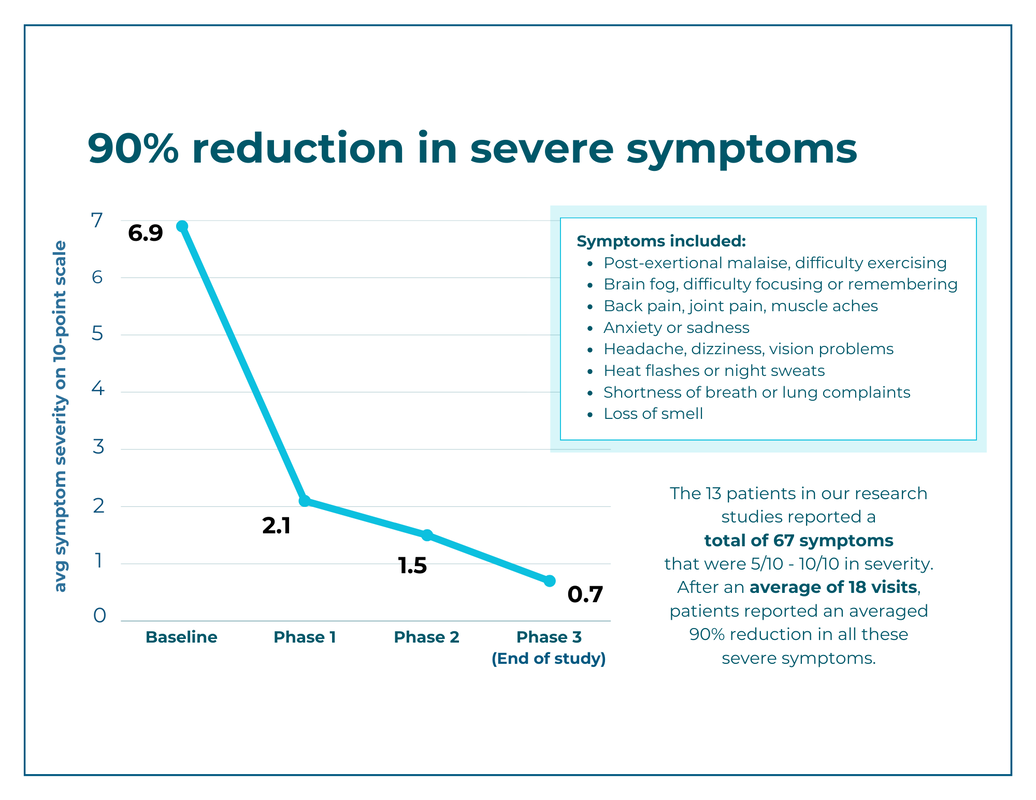
At the start of the study, our 13 participants reported a total of 171 symptoms (other than fatigue), 67 of which were at a severity of 5–10 on a 10-point scale. The graph below shows the average symptom intensity of all 67 severe symptoms at each phase of the treatment protocol. Average intensity went from 6.9/10 to 0.7/10, a decrease of 90%.
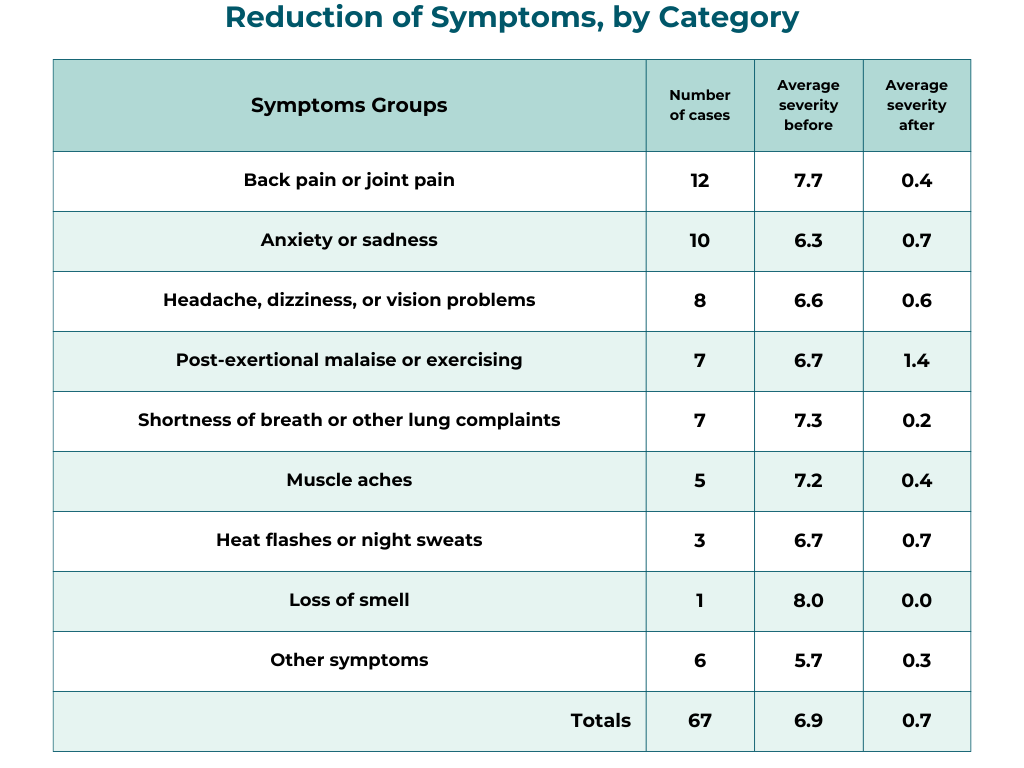
The table below shows the average intensity of the 67 severe symptoms, broken into categories.
At the start of the study, our 13 participants reported a total of 171 symptoms (other than fatigue), 67 of which were at a severity of 5–10 on a 10-point scale. The graph below shows the average symptom intensity of all 67 severe symptoms at each phase of the treatment protocol. Average intensity went from 6.9/10 to 0.7/10, a decrease of 90%.
The table below shows the average intensity of the 67 severe symptoms, broken into categories.